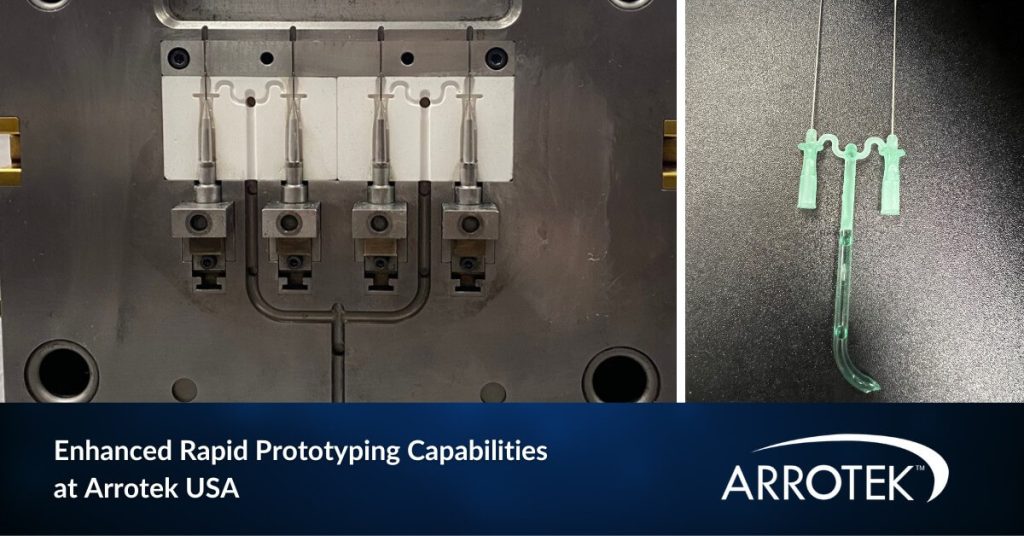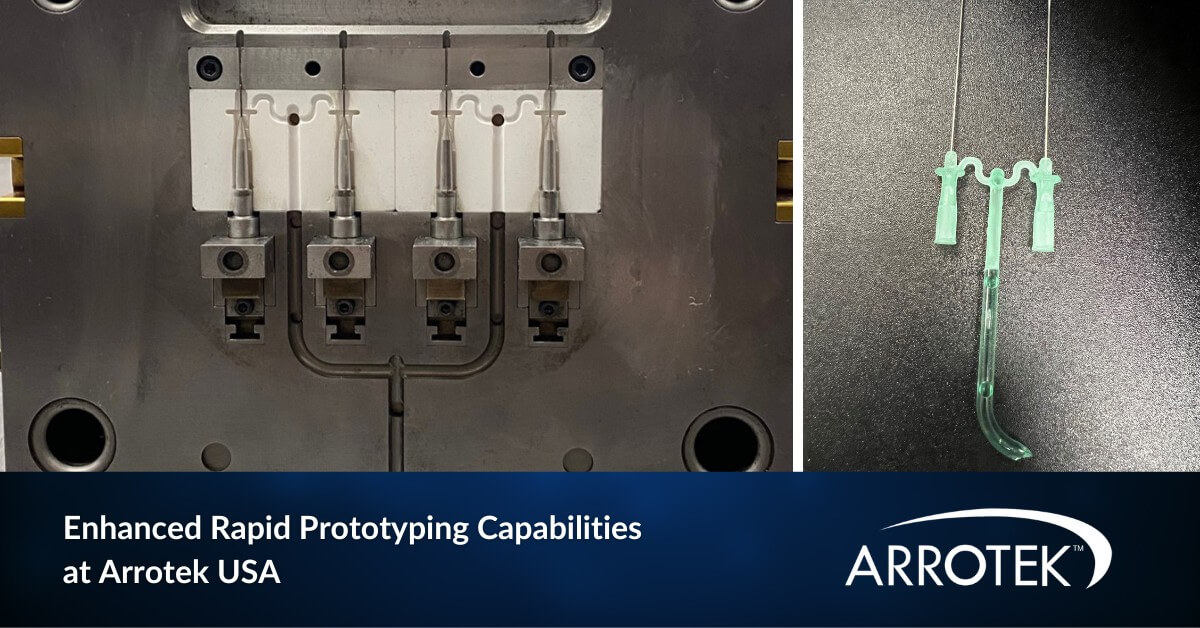
Enhanced Rapid Prototyping Capabilities Available at Arrotek’s US Facility
We have enhanced our medical device rapid prototyping capabilities at our US facility in Massachusetts. These increased capabilities will enable

We have enhanced our medical device rapid prototyping capabilities at our US facility in Massachusetts. These increased capabilities will enable