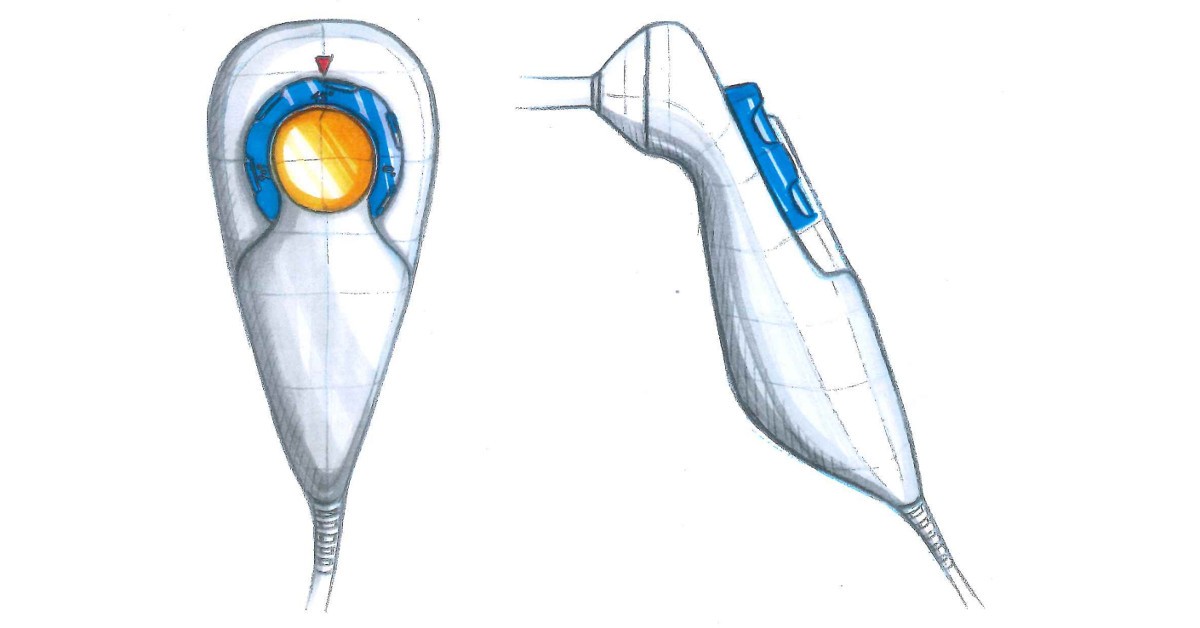
While each new medical device product design and development project is different, the process followed is typically well-structured. There are many reasons for this, not least the importance of ensuring the product is safe to use as well as ensuring both the product itself and the design process adheres to medical device regulations.
At Arrotek, we have a well-established medical device design and product development process. It is outlined below.
Before going through it, however, it may also be helpful to read our blog on the Considerations that Inform and Guide the Medical Device Product Design Process. Both blogs together will give you a holistic view of medical device development from all main perspectives.
Before the Design and Product Development Process Begins
There are two steps you should go through before you get into the full-blown design and development process. You can go through these steps yourself, or you can get the help of an experienced medical device product developer – like us at Arrotek.
The two steps are:
- Identify a need in the market
- Check any existing intellectual property
Identify a Need in the Market
Designing a new medical device product is only part of the journey of making the product a success. It also needs buyers/users in the market, and you need to be able to reach those buyers. This involves identifying the need in the market that your new idea for a medical device will meet.
Remember, developing a new medical device product isn’t just about creating something completely new that nobody has seen or tried before. In fact, many of the most successful medical devices are improvements on existing ideas.
Therefore, it’s crucial to focus on ensuring your idea meets the real needs (rather than perceived needs) of the market.
Check Any Existing Intellectual Property
Any existing intellectual property on the idea you have, or something similar to your idea, could prevent you from bringing your idea to market. The earlier you know about this, the better.
The Process for Designing and Developing New Medical Devices
1. Specification
This includes creating two lists:
- A list of critical requirements
- A list of aspirational requirements
This step also involves developing a design brief for the product. It’s also important to classify the device to ensure the correct regulations are followed during the next steps.
The classification will depend on where you plan to market the device. In the US, for example, the following FDA classifications apply:
- Class I – for simple designs and products that carry very little or no risk
- Class II – for products with a more complicated design and that pose some risk to users
- Class III – for intricate designs and products that carry the greatest amount of risk
2. Concept Development
Using the design brief, your designer will generate concepts for the new product you want to develop. This involves creating a series of sketches to form a storyboard.
In other words, this step is about visualisation of the idea and exploring what is possible as well as the best way to achieve the desired outcome.
3. Develop 3D CAD Models
After you and the designer reach agreement on which concept to pursue, 3D CAD models and detailed drawings of the product are developed.
4. Prototype Production
This step involves the production of a first prototype. This prototype can be manufactured using a range of techniques depending on the product being produced. This includes:
- Balloon blowing
- Tipping
- Laser welding
- Injection moulding
- Bonding
- 3D printing
- Film welding
The final product is then assembled so it can be analysed and assessed.
5. Design Iterations to Refine the Product
This step is about learning from the first prototype to refine the design and improve the product. It involves concept enhancement to create a new and improved version utilising an iterative design process.
6. Prototype Production
In this step, a final prototype is produced. It is typically manufactured in a small batch to allow for the product to be tested and evaluated.
Going Through the Product Design and Development Process
As you can see, the process for developing a new medical device product is quite involved with many different elements and factors to consider. It’s critically important, therefore, that you work with a design team with experience and a proven track record. This will give you the best chance of success.